 Botany Botany
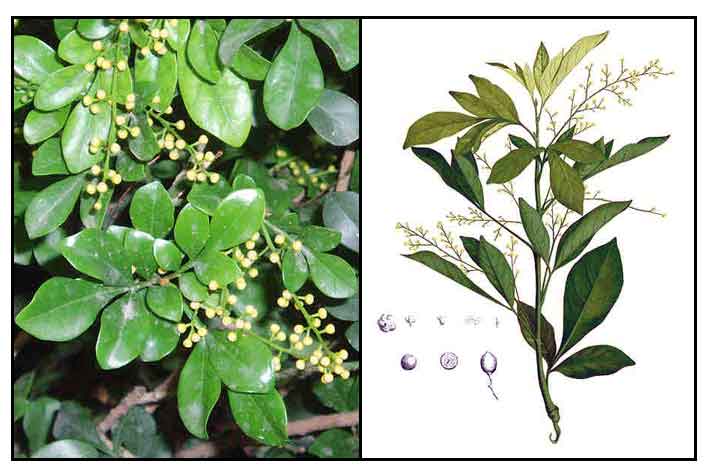
Sinamomong-sungson is a small, much-branched, smooth tree growing from 4 to 7 meters high. Leaves are 5 to 12 centimeters long, with the rachis slightly winged. Leaflets are five, obovate to oblong, 2 to 7 centimeters long, the lower ones being smaller than the upper. Flowers, borne on axillary, lax panicles, 5 to 10 centimeters long, numerous, yellow, very fragrant, and about 3 millimeters in diameter. Fruit is ovoid or subglobose, about 12 millimeters long.
 Distribution Distribution
- Cultivated as an ornamental tree for its fragrant flowers.
- Introduced from southeastern Asia.
Constituents
- Branches and leaves yield triterpenoids (aglaiol, aglaiondiol, aglaitrioland aglaione), alkaloids (odorine and odorinol). Flowers yield volatile oil consisting of a-humulene, ß-caryophyllene, ß-cubebene, ß-gurjunene.
-
Essential oils from the flowers of Aglaia odorata yielded 21 components: hendecane, linalool, decyladehyde, copaene, β-caryophyllend, β-humulene, β-elemene, β-selinene, humuladienone, humulene epoxide Ⅰ, tridecanic acid methyl ester, β-humulene-7-ol, β-humu- lene-7-ol acetate, juniper camphor, heptadecane, khusol acetate, oetadecane, nonadecane, eicosane, heneicosane and docosane, among which β-humnlene-7-ol is expressive of a graceful odor of Aglaia odorata. (6)
- Study isolated of one new coumarino-lignoid, 8-(7′,8′,9′-propanetriol-4′-methoxy-3′-O-phenylpropanoid)-7-hydroxy-6-methoxycoumarin (1), and nineteen known compounds (2–20).
(13)
- Study of leaves yielded a new dammarane triterpene, 3-acetoxy aglinin C, and a new aglain, 10-oxo-aglaiflorin D, along with five known compounds, 3-7. (see study below)
(15)
- GC-MS analysis of essential oil obtained by hydrodistillation of stems identified 39 compounds, representing 76.4% of the oil. Major components were germacrene D (20.3%), α-humulene (12.7%) and ß-caryophyllene (10.2%). Ar-turmerone (1) and eichlerlalactone (2) were isolated from the stem oil and ethanolic stem extract, respectively. (see study below) (18)
Properties
- Roots and leaves considered pectoral, stimulant, febrifuge, tonic and anti-convulsive.
- Flowers are very fragrant in the evening. -
- Studies have shown anti-viral, cytotoxic, anticancer, herbicidal, insecticidal, anti-inflammatory properties.
Parts used
Roots, flowers, leaves.
Uses
Edibility
- Dried flowers used for scenting tea.
- Tender young leaves cooked and eaten as vegetable.
Folkloric
- In the Philippines, decoction of roots and leaves used as a tonic.
- Infusion of flowers given as a cooling drink for eruptive fevers.
- Sino-Annamites used the roots and leaves as pectoral, febrifuge, and tonic; also used for convulsions.
- In China, flowers, leaves, and roots used as a tonic. Branches and leaves used for rheumatic pains, toxic swelling, and superficial infections.
- In Java, infusion of leaves taken as tonic for excessive menses and for venereal diseases.
- In Malaysia, infusion of flowers used to reduce fevers.
Others
Herbicide: Flower pellets show potential as organic herbicide for control of barnyard grass weed.
Perfume: Dried flowers are used to perfume clothes and cigarettes, and to scent teas.
Studies
• Cyclopentabenzofuran Lignan Protein Synthesis Inhibitors: Study isolated rocaglaol, known compound pyrimidinone and the novel compound aglaiastatin from the CHCl3 extract of leaves. The three were potent inhibitors of the growth of K-ras-NRK cells. (2)
• Dolabellane Diterpenoids / Cytotoxicity: Study yielded 5 dolabellane diterpenoids; two showed weak cytotoxicity against human myeloid leukemia HL-60, hepatocellular carcinoma, and lung cancer A-549 cells.
• Anti-Herpes Simplex Virus Type 1: Of 20 Thai medicinal plants evaluated for anti-herpes simplex virus 1 activity, eleven, including A odorata, inhibited plaque formation of HSV-1 more than 50%. AO was also effective against thymidine-kinase-deficient HSV-1 and phosphonoacetate-resistant HSV-1 strains. It showed a potential as anti-HSV1 agent. (3)
• Elemene & Cisplastin Synergy / Anti-Cancer: Elemene (1-methyl-1-vinyl-2.4-diisopropenylcyclohexane) has been extracted from numerous plants, including the flowers and leaves of Aglaia odorata. Article describes the synergy of the combination of cisplastin with b-elemene in in vitro assays against androgen-insensitive prostate cancer cells. B-elemene as an anticancer drug has anti-tumor activity against a broad spectrum of cancers, with low level of toxicity. (4)
• Insecticidal / Rocaglamide: Organic extracts of the twigs and leaves of Aglaia odorata yielded eight insecticidal cyclopentatetrahydrobenzofuran rocaglamide derivatives. The isolated rocaglamide derivatives exhibited strong insecticidal activity towards neonate larvae of the polyphagous pest insect Spodophera littoralis when incorporated into artificial diet. (5) Rocaglamide, a highly substituted benzofuran, an active insecticidal constituent in the twigs of A. odorata, inhibits larval growth and is insecticidal to both variegated cutworms, Peridroma saucia and Asian armyworms, Spodopteraa litura. (19)
• Herbicidal Efficiency: Study evaluated eighteen species of Thai local plants as botanical herbicides for reducing the use of harmful herbicides in agricultural pest management. Aglaia odorata leaf extracts demonstrated the highest germination inhibitory effect against Mimosa seedling. Results suggest A. odorata leaf extract may be a potential natural resource as a botanical herbicide to reduce the use of harmful ones.
(8)
• Cyclopentabenzofuran / Lignan Protein Synthesis Inhibitors: A CHCl3 extract of leaves yielded Ras function inhibitors, rocaglaol and related compounds, pyridinone and the novel aglaiastatin. The three compounds were potent inhibitors of growth of K-ras-NRK cells. They also specifically inhibited protein synthesis. Aglastatin reduced the amount of Ras, possibly through inhibition of de novo synthesis. (9)
• Insecticidal Active Constituents / Twigs: Study evaluated the insecticidal active constituents from twigs. Seven compounds were isolated: rocaglamide, desmethyl rocaglamide, 8-methoxymarikarin, 7-hydroxy-6-methoxy-coumarin , 3′-hydroxy-methylrocaglate, 3′-hydroxyrocaglamide, and marikarin. (10)
• Diterpenoids and Triterpenoids / Anti-Inflammatory / Leaves: Study of leaves isolated five compounds: two dolabellane diterpenoids, two dammarane triterpenoids, and a protostane triterpenoid, along with 20 known compounds. On evaluation for anti-inflammatory activity, eleven compounds exhibited potent nitric oxide inhibitory activity with IC50 ranging from 2.1 to 14.2 µM, and three compounds showed significant activity against PGE1 release with IC50 of 2.6, 16.1 and 23.0 µM. (14)
• Cytotoxicity / Human Cancer Cell Lines / Leaves: Study yielded a new dammarane triterpene, 3-acetoxy aglinin C, and a new aglain, 10-oxo-aglaiflorin D, along with five known compounds, 3-7. Compounds 1-7 were assayed for cytotoxicity towards human lung cancer cell line (AGZY83-a) and human liver cancer cell line (SMMC-7721). Compound 6, rocaglaol, exhibited distinctive antiproliferative activities against the two cell lines with IC50 of 0.03 µM and 3.62 µM, respectively. Compound 7 showed strong activity against SMMC-7721 cells with IC50 of 10.69 µM. (15)
• Phytotoxic Substance / Rocaglaol: Study investigated the allelopathic properties and phytotoxic substances in A. odorata. Aqueous EtOH extracts of leaves have inhibited root and shoot growth of garden cress, lettuce, alfalfa, timothy and ryegrass. Study isolated rocaglaol which showed an inhibitory effect on weed species E. crus-galli much greater than that of abscisic acid. Results suggest rocaglaol may be a major contributor to the allelopathic effect of A. odorata and the bioherbicide PORGANIC. (16)
• Odorine and Odorinol / Cancer Chemopreventive: Study isolated aminopyrrolidine-diamides, odorine and odorinol from Aglaia odorata. The compounds exhibited potent anti-carcinogenic effects in a two-stage carcinogenesis test of mouse skin induced by DMBA as an initiator and TPA as a promoter. Study showed odorine and odorinol inhibited both the initiation and promotion stages of two-stage skin carcinogenesis. (17)
• Antimicrobial / Essential Oil / Stem: GC-MS study of essential oil from stems yielded 39 compounds. Essential oil and ethanol extract were tested against gram-positive and gram-negative bacterial strains including B. cereus, S. aureus, A. baumannii and E. coli, as well as three rice fungal pathogens. The oil and ar-turmerone (1)exhibited significant activity against the three rice pathogens. Eichlerlalactone (2) exhibited good antibacterial activity against Gram-positive test pathogens. (see constituents above) (18)
Availability
Wild-crafted.
|

![]()



 Botany
Botany
