
General info
- Sphaeranthus is a genus of Asian, African, and Australian plants in the tribe Inuleae within the family Asteraceae.
- Etymology:
The genus name Sphaeranthus derives from Greek words sphair (globe-shaped: the heads crowded into a globose glomerule; in globular clusters) and anthos (flower). The species epithet is africanus is Latin, meaning 'relating to Africa'. (18)
 Botany Botany
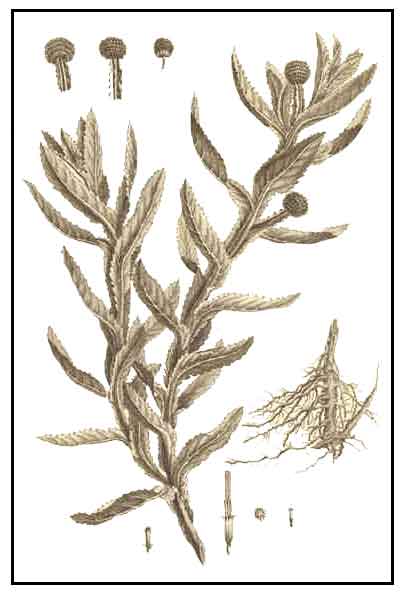
• Botobotonisan is a more or less hairy or nearly smooth, rather coarse, erect or spreading, branched herb less than 1 meter in height. Stems and branches are prominently winged with three thin, wide, longitudinal structures which are the extension of the leaf blades. Leaves are obovate to oblong-obovate, 4 to 13 centimeters long, without stalks, and finely toothed at the margins. Heads are numerous, borne in dense, rounded clusters about 1 centimeter in diameter, and occur singly at the ends of erect, winged stalks. Flowers are greenish-white.
Distribution
- Native to the Philippines.
-
In open, rather damp waste places in and about towns at low and medium altitudes throughout the Philippines.
- Abundant in rice fields.
- Also native to Andaman Is., Bangladesh, Borneo, Cambodia, China South-Central, China Southeast, Hainan, India, Jawa, Kenya, Lesser Sunda Is., Madagascar, Malaya, Mozambique, Myanmar, Nepal, Nicobar Is., Northern Territory, Queensland, Sri Lanka, Taiwan, Tanzania, Thailand, Vietnam, Western Australia. (6)
Constituents
- Study yielded seven compounds: stigmasterol, chrysophenol D, 3,7-dimethoxy-4',5,6-trihydroxyflavone, chrysophenol C, 3alpha, 5beta-diangeloxoyloxy-7-hydroxycarvotanacetone, l-angeloxoyloxy-3-[4'-angeloxoyloxy-3'-methoxy]-2-propene and 1-angeloxoyloxy-3-[4'-isopentanoloxoyloxy-3'-methoxy]-2-propene.
 - Flowers contains a volatile oil. - Flowers contains a volatile oil.
- Essential oil yields methyl chavicol, α-ionone, d-cadinene, p-methoxy cinnamaldehyde as major constituents and α-terpinene,citral, geraniol, geranyl acetate, ß-ionene, sphaerene as minor constituents.
- Dichlormethane extract of leaves yielded carvotanacetone derivatives (1, 3a, and 3b), chrysosplenol D (4), squalene (5) spinasterol (6) and stigmasterol (7). (see study below) (7)
- Study of ethanolic extract of whole plant yielded seven compounds, namely: stigmasterol, chrysophenol D, 3,7-dimethoxy-4',5,6-trihydroxyflavone, chrysophenol C, 3alpha, 5beta-diangeloxoyloxy-7-hydroxycarvotanacetone, l-angeloxoyloxy-3-[4'-angeloxoyloxy-3'-methoxy]-2-propene and 1-angeloxoyloxy-3-[4'-isopentanoloxoyloxy-3'-methoxy]-2-propene.
- Study of aerial parts isolated five carvotacetone derivatives, including two known ones, 3,5-diangeloyloxy-7-hydroxycarvotacetone (1) and 3-angeloyloxy-5-[2″,3″-epoxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (2), along with three new compounds, 3-angeloyloxy-5-[3″-chloro-2″-hydroxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (3), 5-angeloyloxy-7-hydroxy-3-tigloyloxycarvotacetone (4), and 3-angeloyloxy-5,7-dihydroxycarvot-acetone (5). (12)
-
A previous study isolated five carvotacetone (1-5). Study isolated two diastereomeric carvotacetones (3-angeloyloxy-5-[2″S,3″R-dihydroxy-2″-methyl-butanoyloxy]-7-hydroxycarvotacetone (6) and 3-angeloyloxy-5-[2″R,3″R-dihydroxy-2″-methyl-butanoyloxy]-7-hydroxycarvotacetone (7), asperglaucide (8) and chrysoplenol D (9). (see study below) (13)
- Study isolated a new carvotacetone sphaeranthone A and four known compounds, 3-angeloyloxy-5-[2″,3″-epoxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (2), 3-angeloyloxy-5-[3″-chloro-2″-hydroxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (3), chrysosplenol D (4), and 3-O-methylquercetin (5) from the leaves of S. africanus. (see study below) (17)
Properties
- Anthelmintic, stomach tonic and stimulant, antiblenorrhagic.
- Bitter, aromatic, vermifuge, diuretic, emollient, resolvent.
- In Ayurveda, considered laxative, digestible, tonic, fattening, alterative, anthelmintic and alexipharmic.
- Plant has the drug odor of terebenthine which is transmitted to the urine and sweat.
- Studies have shown antioxidant, cytotoxic, anti-inflammatory, phytoremediative, antifungal, and antibacterial, anti-inflammatory, anti-proliferative, antidiabetic, α-glucosidase inhibitory properties.
Parts used
Leaf juice, leaves, tops.
Uses
Edibility
- Leaves used as pot-herb.
Folkloric
- Used as anthelmintic, as powder, 2 to 4 grams, with a little molasses or syrup.
- Bitter and aromatic, used for diseases of the stomach and intestines for tonic and stimulant effect.
- Decoction of leaves and tops used as stomach tonic and also employed as antiblenorrhagic.
- In Ayurveda, plant pacifies vitiated vata, pitta epilepsy, migraine, jaundice, fever, cough, hemorrhoids, helminthiasis, skin diseases.
- In Bengal, plant used as tonic, vermifuge, and diuretic.
- In Indo-China, used as emollient and resolvent; applied as poultice to any ailing body part.
- Juice of leaves used as gargle in inflammation of the throat.
- Plant used as aphrodisiac. (4)
- In Vietnamese traditional medicine, used for treatment of sore throat, pain and swelling.
(12)
Others
- Aqueous extract reported as poisonous to American cockroaches.
- Plant used as soil fertility indicator.
Studies
• Free Radical Scavenging Activity / Cytotoxicity: Study of ethanolic crude extract of whole plant exhibited free radical scavenging activity and cytotoxicity against various carcinoma cell lines.
• Carvotanacetone Derivatives / Antibacterial / Antifungal: Dichlormethane extract of air-dried leaves of Sphaeranthus africanus yielded four new carvotanacetone derivatives. Some compounds showed antibacterial activity against S. aureus and P. aeruginosa and antifungal activity against C. albicans, T. mentagrophytes and A. niger. (3)
•
Biologic Activities of Constituents: Study of dichlormethane extract of leaves yielded constituents with known biologic activities. Chrysosplenol D, squalene, spinasterol and stigmasterol have been reported to have anticancer activities. Stigmasterol is known to lower plasma cholesterol and inhibit intestinal cholesterol absorption. Squalene is known to significantly suppress colonic ACF formation and crypt multiplicity, while also known to have cardioprotective effects via antioxidant and hypolipidemic properties. (see constituents above) (7)
• Cytotoxicity / Anti-Inflammatory / Aerial Parts: Study evaluated various extracts of aerial parts of Sphaeranthus africanus for cytotoxic activity using various cancer cell lines and anti-inflammatory activity via COX-2 expression in THP1 monocytes. The n-hexane and dichlormethane extracts showed cytotoxic activity. Two compounds were identified as carvotanacetone derivatives. (8)
• Remediation Study for Heavy Metal Soil Pollution / Phytostabilization
of Lead: Study showed Sphaeranthus africanus has potential for stabilization of lead. (9)
•
Cytotoxicity / Free Radical Scavenging: Study of an ethanolic extract of whole plant of Sphaeranthus africanus showed cytotoxicity against various carcinoma cell lines. The Isolated flavonoids (chrysophenol D, 3,7-dimethoxy-4',5,6-trihydroxy flavone, chrysophenol C) and 3alpha, 5beta-Diangeloxoyloxy-7-hydroxy carvotanacetone and showed free radical scavenging activity against DPPH radical. (see constituents above) (10)
•
Cytotoxicity of Carvotacetones against Cancer / Apoptotic Potential: Study of aerial parts isolated five carvotacetone derivatives, including two known ones, 3,5-diangeloyloxy-7-hydroxycarvotacetone (1) and 3-angeloyloxy-5-[2″,3″-epoxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (2), along with three new compounds, 3-angeloyloxy-5-[3″-chloro-2″-hydroxy-2″-methylbutanoyloxy]-7-hydroxycarvotacetone (3), 5-angeloyloxy-7-hydroxy-3-tigloyloxycarvotacetone (4), and 3-angeloyloxy-5,7-dihydroxycarvot-acetone (5). All compounds exhibited significant antiproliferative activity against four tested human cancer cell lines i.e., CCRF-CEM, MDA-MB-231, U-251, and HCZT-116. Compound 3 and 4 showed highest activity with IC50s ranging from 0.6 to 2.9 µM and 1.3 to 2.5 µM, respectively. The compounds also showed inhibitory activity toward HEK-293 human embryonic kidney cells with IC50s from 2.5 to 5.5 µM. (12)
•
Anti-Inflammatory / Antiproliferative: A previous study (12) isolated five carvotacetones that displayed cytotoxicity against several cancer cell lines. Study evaluated the anti-inflammatory activity and anti-proliferative activity of isolated compounds. COX-1 and COX-2 assays of compounds 1-9 showed compounds 1 and 2 possess potent and selective COX-2 inhibitory activity with IC50s of 3.6 and 0.5 µM, respectively. COX-2 gene expression assay showed some carvoacetones exhibited inhibitory effects on COX-2 gene expression in THP-1 macrophages. Compound 4 was most activity in inhibiting synthesis of COX-2 by 55% at 2.06 µM. In iNOS assay, all seven carvoacetones inhibited NO production. XTT assay of newly isolated compounds revealed two isomeric carvoacetones (6,7) exhibited considerable antiproliferative activity against four cancer cell lines (CCRF-CEM, MDA-MB-231, HCT-116, U-251) with IC50s range of 1.23 to 8.o µM. (see constituents above) (13)
•
Antidiabetic / Antioxidant / Cytotoxic / Aerial Parts: Study evaluated hydrophilic extracts of aerial parts for antioxidant and antidiabetic properties and roots for cytotoxic activity. All extracts inhibited lipid peroxidation with IC50s range from 2.05 to 3.56 µg/mL. The 50% ethanol extract exhibited most potent
α-glucosidase inhibition with IC50 of 4.80 µg/mL. Cytotoxic activity of root extracts was 2- to 5-fold less than that of aerial parts. Nevertheless, the dichloromethane and ethyl acetate extracts of root showed selective effect on leukemia cells, with no effect of normal HEK-293 cell line. Study suggests antidiabetic and antioxidant potential and anticancer activity for the plant's root. (14)
• α-Glucosidase Inhibitory / Aerial Parts: Study of aerial parts isolated eight compounds. 3-Angeloyloxy-5-[2′′S,3′′S-dihydroxy-2′′-methylbutanoyloxy]-7-hydroxycarvotacetone was a new compound. The isolated compounds showed moderate activity for α-glucosidase inhibition with IC50s ranging from 128.9 to 274.3 µM. (15)
• Antimicrobial / Efflux Pump Inhibitory Activity against Mycobacteria / Carvotacetones: Study isolated and screened carvotacetones (1-7) isolated from S. africanus for antimycobacterial and efflux pump (EP) inhibitory potential against mycobacterial model strains Mycobacterium smegmatis mc2 155, M. aurum ATCC 23366, and M. bovis BCG ATCC 35734. Carvotacetones 1-6 showed potent antibacterial effects on MN. aurum and M. bovis BCG with MIC ≤ 31.25 mg/L and could enhance EtBr activity against M. smegmatis. Compounds 1 and 6 were highly effective against M. aurum and M. bovis BCG. Compound 1 was identified to have potential for mycobacterial efflux mechanisms and a promising adjuvant in the therapy of tuberculosis and non-tubercular mycobacterial infections. (16)
• Sphaeranthone A / α-Glucosidase Inhibitory / Leaves: Study isolated a new carvotacetone sphaeranthone A and four known compounds. Compounds 1-3 showed moderate activity for α-glucosidase activity with IC50s of 103, 146, and 49 µg/mL, respectively. (see constituents above) (17)
Availability
Wild-crafted.
|

![]()




 Botany
Botany
